ДѓЛсЬибћБЈИц Plenary Lectures
ВјНсИпЗжзгСїЬхЗЧЯпадСїБфааЮЊЕФЗжзгЛњРэ ТЌгюдД1ЃЌАВСЂМб1*ЃЌЭѕЪЎЧь2ЃЌЭѕеёИй1,3 1жаЙњПЦбЇдКГЄДКгІгУЛЏбЇбаОПЫљИпЗжзгЮяРэгыЛЏбЇЙњМвжиЕуЪЕбщЪвЃЌГЄДК 130022
*Email: ljan@ciac.ac.cn ИпЗжзгЗЧЯпадСїБфбЇЖдгкИпЗжзгКЭЦфЫќИДдгСїЬхМгЙЄКЭЕїПижСЙиживЊЃЌЦфЗЂеЙЖдЪЏгЭЛЏЙЄЁЂЩњЮявНвЉЕШСьгђгаживЊЕФжЇГХзїгУЁЃУшЪіВјНсИпЗжзгСїЬхеГЕЏаджЪЕФОЕфФЃаЭЃЈЙмзгФЃаЭЃЉПЩвдКмКУЕидЄВтВјНсИпЗжзгСїЬхЕФЦНКтЬЌКЭНќЦНКтЬЌаджЪЁЃЕЋЪЧЃЌНќЦкЪЕбщЗЂЯжЕФвЛЯЕСаЕфаЭЗЧЯпадСїБфбЇЯжЯѓШДВЛФмЛљгкЙмзгФЃаЭРДНтЪЭЁЃЮЊСЫбщжЄЙмзгФЃаЭЕФЛљБОМйЖЈКЭЮяРэЭМЯёЃЌНвЪОЗЧЯпадСїБфбЇЯжЯѓЕФЗжзгЛњРэЃЌЮвУЧЗЂеЙКЭНЈСЂСЫвЛЬзбаОПВјНсИпЗжзгСїЬхЕФBrownЖЏСІбЇФЃФтКЭЗжЮіЗНЗЈЃЌЗЂЯждкПьЫйДѓаЮБфЬѕМўЯТЃЌИпЗжзгСДдЫЖЏВЛЗўДгRouseЖЏСІбЇЃЛдкЦєЖЏМєЧаГѕЦкЃЌБЛРЩьЕФЁАВјНсЭјТчЁБФмЙЛвжжЦИпЗжзгСДНтВјНсЃЛЩЯЪіНсЙћЪзДЮдкЗжзгЫЎЦНЩЯжЪвЩСЫЙмзгФЃаЭЛљБОМйЖЈКЭЮяРэЭМЯёЕФгааЇадЃЛЭЌЪБЃЌЬсГіВЂжЄУїСЫШ§ИіЕфаЭЕФВјНсИпЗжзгСїЬхЗЧЯпадСїБфбЇЯжЯѓЃЈЦєЖЏМєЧаЯТЕФЁАгІСІЙ§ГхЁБЃЌНздОМєЧаКѓЕФЁАКъЙлСїЖЏЁБКЭЁАЕЏадЛжИДЁБЃЉЕФЗжзгЛњРэЁЃдкДЫЛљДЁЩЯЃЌЬсГіСЫЁАВјНсИпЗжзгСїЬхМєЧавжжЦНтВјНсЁБЕФаТИХФюЃЌгУгкУшЪіДЋЭГРэТлЮоЗЈРэНтЕФВјНсИпЗжзгСїЬхЗЧЯпадСїБфбЇааЮЊЃЌВЂЭЈЙ§ФЃаЭЛЏМЦЫугыРэТлЗжЮіЃЌНЋИУИХФюНјааСЫЭъЩЦЁЃЮвУЧЕФЙЄзїЮЊжиаТЙЙНЈВјНсИпЗжзгСїЬхЗЧЯпадСїБфбЇРэТлКЭЗЂеЙИпЗжзгВФСЯМгЙЄаТММЪѕЬсЙЉСЫЧхЮњЕФЮяРэЭМЯёЁЃ
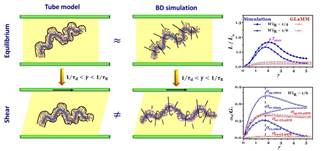
Fig. 1 Comparison of the physical picture, the normalized contour length of the primitive chain, and the stress contribution of the entangled polymer chains during fast startup shear from the simulation relative to the schematic and prediction of the tube model ЙиМќДЪЃКЗЧЯпадСїБфбЇЃЛЙмзгФЃаЭЃЛЦєЖЏМєЧаЃЛНздОМєЧаЃЛСДВјНс ВЮПМЮФЯз [1] Lu, Y. Y.; An, L. J.; Wang, S.-Q.; Wang, Z.-G. ACS Macro Lett. 2013, 2: 561.
Molecular Mechanisms for Nonlinear rheological Behaviors of Entangled Polymers Yuyuan Lu1, Lijia An1*, Shi-Qing Wang2, and Zhen-Gang Wang1,3 1 State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China The tube model provides a reasonable and appealing description of the equilibrium and near equilibrium dynamics of entangled polymer melts and concentrated solutions. However, recently emerged experimental observations have revealed new phenomenology that is very difficult for the tube model to explain. Using Brownian Dynamics simulation together with a set of newly developed analysis tools, we systematically examined the evolution of chain conformation and entanglements, as well as the molecular mechanisms for a series of nonlinear rheological phenomena of entangled polymer melts under fast large deformation. Our work for the first time offers convincing evidence that calls into question the fundamental assumption in the prevailing tube model that the topological constraint can be modeled as a Rouse chain confined in a smooth, barrierless tube. The concept of disentanglement inhibition by shear proposed in our work provides novel insight into the molecular mechanism of the large deformation behavior of polymers, which may lead to a new theoretical framework for the nonlinear rheology of entangled polymers. |
||||
|
АВСЂМбЃЌ1964ФъГіЩњЃЌ1992ФъдкМЊСжДѓбЇЛЏбЇЯЕЛёРэбЇВЉЪПбЇЮЛЃЌЭЌФъЗжХфЕНжаЙњПЦбЇдКГЄДКгІгУЛЏбЇбаОПЫљЙЄзїжСНёЁЃ1997ФъБЛЦИЮЊбаОПдБЃЌ2008ФъШЮжаЙњПЦбЇдКГЄДКгІгУЛЏбЇбаОПЫљЫљГЄЁЃ1998ФъЛёЙњМвНмГіЧрФъПЦбЇЛљН№зЪжњЃЌ2006ФъзїЮЊбЇЪѕДјЭЗШЫЛёЙњМвздШЛПЦбЇЛљН№ДДаТбаОПШКЬхПЦбЇЛљН№зЪжњЃЌ2012ФъзїЮЊЪзЯЏПЦбЇМвГаЕЃЙњМв973МЦЛЎЯюФПЃЌ2014ФъШыбЁЙњМвЁАЭђШЫМЦЛЎЁБАйЧЇЭђЙЄГЬСьОќШЫВХЃЌ2015ФъЕБбЁЮЊжаЙњПЦбЇдКдКЪПЁЃЫћвЛжБжТСІгкИпЗжзгЮяРэЛљДЁбаОПЃЌдкИпЗжзгЗЧЯпадСїБфбЇЁЂЬиадеГЖШРэТлЁЂКЌИеадЧЖЖЮЙВОлЮяЮЂЯрЗжРыЕШЗНУцШЁЕУСЫживЊбаОПГЩЙћЁЃЙВЗЂБэТлЮФ330грЦЊЃЌБЛЫћШЫв§гУ3200грДЮЃЛдкЙњФкЭтбЇЪѕЛсвщзїДѓЛсБЈИцКЭбћЧыБЈИц40грДЮЃЛЪкШЈжаЙњЗЂУїзЈРћ9МўКЭУРЙњЗЂУїзЈРћ3МўЁЃ ПЮЬтзщЭјжЗЃКhttp://pts.ciac.jl.cn/ |
|||
жаЙњЛЏбЇЛсУиЪщДІ
|
ЕиЁЁЁЁжЗЃКББОЉЪажаЙиДхББвЛНж2КХ |
|
|
ИіШЫЛсдБЃКqiaoqinzhao@iccas.ac.cn |
бЇЪѕНБРјЃКyuehe@iccas.ac.cn |